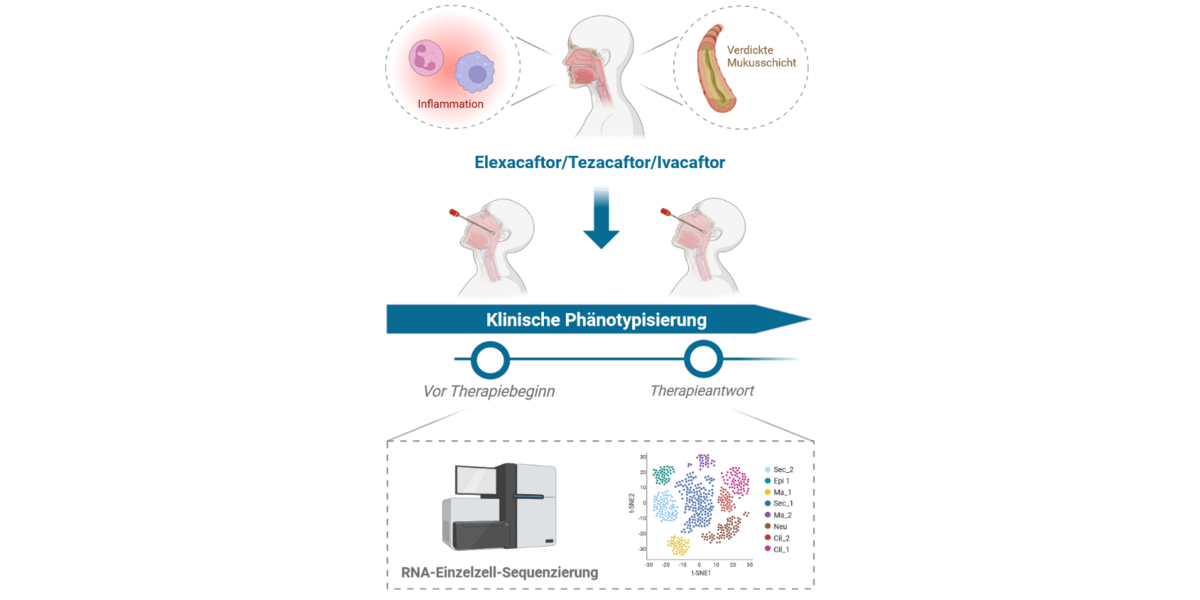
The new CFTR modulator therapy with elexacaftor, tezacaftor, and ivacaftor (ETI) has demonstrated convincing efficacy in most cystic fibrosis (CF) patients. These patients feel significantly better after the therapy, and experience improvements in their sweat chloride levels. However, it has also been found that some CF patients respond very differently to the therapy – even those who carry the same CFTR mutations, such as the F508del mutation. The scientists suspect the reason for this lies at the cellular level, but how the therapy affects individual cells has not yet been studied in detail. It is precisely this question that the research groups led by Dr. Saskia Trump of the BIH and Dr. Simon Gräber now want to answer. The German Cystic Fibrosis Association (Mukoviszidose e.V.) is providing the project with €188,000 in funding. It supports a wide range of projects, from basic medical research to clinical studies, with the aim of improving therapeutic options and the quality of life of CF patients.
CF patients have a mutation in the CFTR gene that renders the CFTR chloride channel in the cell membrane partially functional or completely non-functional. This defect causes the symptoms typical of cystic fibrosis, such as the accumulation of viscous mucus in the lungs or intestines. The main aim of CFTR modulators is to partially restore the function of the CFTR channel in the cell membrane. Initial studies of CFTR modulator therapy indicate that these drugs may also affect immune cells.
Using transcriptome analyses to investigate therapeutic effects
The focus of the project is to examine the effects of the triple combination of ETI on mucosal and immune cells in the upper respiratory tract. In order to gain an understanding of the molecular processes at the cellular level, the scientists plan to analyze the transcriptome (the sum of all genes being expressed and transcribed into mRNAs) of single cells from different patients. This will involve using nasal swabs to collect epithelial and immune cells from CF patients before and three months after commencement of the ETI therapy. They will then isolate the mRNA – the transcriptomes – from each cell, analyze them, and compare them with those of healthy control subjects. In this way, they hope to find out how ETI therapy influences the gene expression of CF patients.
Improving the therapeutic success of modulator therapy in all patients
The objective is to identify patterns associated with a strong or weak response to ETI therapy. If successful, the findings could on the one hand help define new biomarkers that enable the prediction of whether a patient will likely respond very well to ETI therapy or tend to have a much weaker response. On the other hand, it may also be possible to find out what causes the therapy to be very effective in one case but not in another, thus further advancing personalized medicine for CF patients.